Review Can You Label the Components of Replicating Dna Strands?
Arthur Kornberg compared Dna to a record recording of instructions that can be copied over and over. How do cells brand these virtually-perfect copies, and does the procedure ever vary?
Scientists take devoted decades of effort to understanding how deoxyribonucleic acrid (Deoxyribonucleic acid) replicates itself. In simple terms, replication involves use of an existing strand of Deoxyribonucleic acid as a template for the synthesis of a new, identical strand. American enzymologist and Nobel Prize winner Arthur Kornberg compared this process to a tape recording of instructions for performing a job: "[E]xact copies can exist made from it, as from a tape recording, and so that this information can be used again and elsewhere in time and space" (Kornberg, 1960).
In reality, the process of replication is far more than circuitous than suggested by Kornberg's analogy. Researchers typically utilize simple bacterial cells in their experiments, just they still do not have all the answers, peculiarly when information technology comes to eukaryotic replication. However, scientists are familiar with the basic steps in the replication process, and they continue to rely on this information as the basis for continued research and experimentation.
The Molecular Machinery of Bacterial Dna Replication
A typical bacterial cell has anywhere from most 1 million to 4 meg base pairs of Dna, compared to the 3 billion base of operations pairs in the genome of the mutual house mouse (Mus musculus). Still, even in bacteria, with their smaller genomes, Dna replication involves an incredibly sophisticated, highly coordinated serial of molecular events. These events are divided into four major stages: initiation, unwinding, primer synthesis, and elongation.
Initiation and Unwinding
During initiation, so-chosen initiator proteins bind to the replication origin, a base-pair sequence of nucleotides known equally oriC. This binding triggers events that unwind the Dna double helix into ii single-stranded DNA molecules. Several groups of proteins are involved in this unwinding (Effigy one). For instance, the Dna helicases are responsible for breaking the hydrogen bonds that join the complementary nucleotide bases to each other; these hydrogen bonds are an essential feature of James Watson and Francis Crick'south three-dimensional DNA model. Considering the newly unwound single strands have a trend to rejoin, another group of proteins, the unmarried-strand-binding proteins, go along the single strands stable until elongation begins. A third family of proteins, the topoisomerases, reduce some of the torsional strain caused by the unwinding of the double helix.
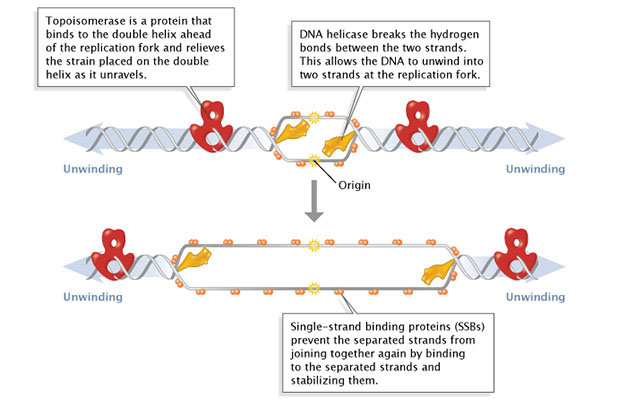
Effigy 1: Facilitation of Dna unwinding.
During Deoxyribonucleic acid replication, several proteins facilitate the unwinding of the Dna double helix into two unmarried strands. Topoisomerases (red) reduce torsional strain caused by the unwinding of the Deoxyribonucleic acid double helix; Deoxyribonucleic acid helicase (yellow) breaks hydrogen bonds between complementary base-pairs; single-strand binding proteins (SSBs) stabilize the separated strands and forbid them from rejoining.
© 2014 Nature Education Adapted from Pierce, Benjamin. Genetics: A Conceptual Approach, 2nd ed. All rights reserved. ![]()
As previously mentioned, the location at which a Deoxyribonucleic acid strand begins to unwind into two split up single strands is known as the origin of replication. As shown in Figure one, when the double helix unwinds, replication proceeds along the 2 single strands at the aforementioned time but in opposite directions (i.e., left to right on one strand, and right to left on the other). This forms 2 replication forks that move along the DNA, replicating equally they go.
Primer Synthesis
Primer synthesis marks the beginning of the actual synthesis of the new Deoxyribonucleic acid molecule. Primers are short stretches of nucleotides (virtually ten to 12 bases in length) synthesized by an RNA polymerase enzyme called primase. Primers are required considering Dna polymerases, the enzymes responsible for the bodily addition of nucleotides to the new Dna strand, can only add together deoxyribonucleotides to the three'-OH group of an existing chain and cannot begin synthesis de novo. Primase, on the other paw, can add together ribonucleotides de novo. Later, after elongation is complete, the primer is removed and replaced with DNA nucleotides.
Elongation
Finally, elongation--the add-on of nucleotides to the new Dna strand--begins subsequently the primer has been added. Synthesis of the growing strand involves adding nucleotides, one by one, in the verbal order specified by the original (template) strand. Recall that one of the key features of the Watson-Crick DNA model is that adenine is always paired with thymine and cytosine is always paired with guanine. So, for example, if the original strand reads A-G-C-T, the new strand will read T-C-G-A.
DNA is always synthesized in the 5'-to-3' direction, pregnant that nucleotides are added only to the iii' end of the growing strand. As shown in Figure 2, the five'-phosphate group of the new nucleotide binds to the 3'-OH group of the last nucleotide of the growing strand. Scientists take however to identify a polymerase that tin can add together bases to the five' ends of Dna strands.
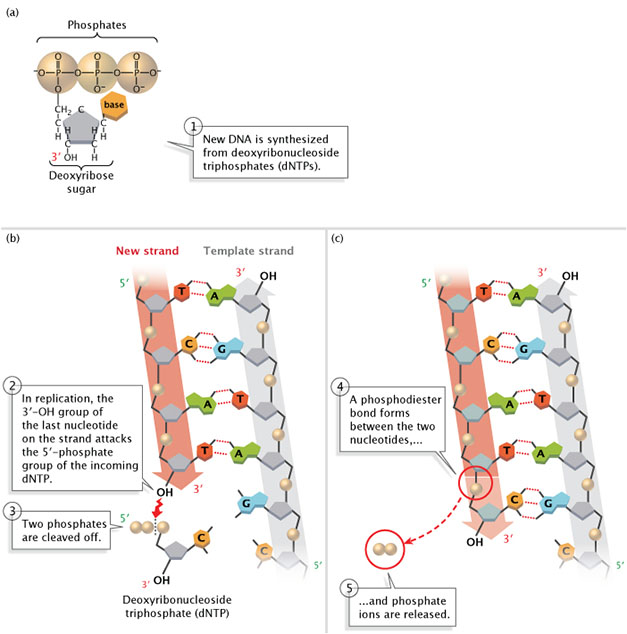
Figure 2: New DNA is synthesized from deoxyribonucleoside triphosphates (dNTPs).
(A) A deoxyribonucleoside triphosphate (dNTP). (B) During Deoxyribonucleic acid replication, the iii'-OH group of the last nucleotide on the new strand attacks the 5'-phosphate grouping of the incoming dNTP. Two phosphates are cleaved off. (C) A phosphodiester bail forms betwixt the two nucleotides, and phosphate ions are released.
© 2014 Nature Instruction Adapted from Pierce, Benjamin. Genetics: A Conceptual Approach, 2nd ed. All rights reserved. ![]()
The Discovery of Deoxyribonucleic acid Polymerase
While studying Due east. coli bacteria, enzymologist Arthur Kornberg discovered that Dna polymerases catalyze DNA synthesis. Kornberg's experiment involved mixing all of the bones "ingredients" necessary for Due east. coli Deoxyribonucleic acid synthesis in a test tube, including nucleotides, Due east. coli extract, and ATP, so purifying and testing the enzymes involved. Using this method, Kornberg not but discovered Deoxyribonucleic acid polymerases, just he likewise performed some of the initial work demonstrating how enzymes add new nucleotides to growing DNA bondage (Kornberg, 1959).
Scientists take since identified a total of five different DNA polymerases in E. coli, each with a specialized role. For example, DNA polymerase Iii does well-nigh of the elongation work, adding nucleotides one past 1 to the 3' end of the new and growing single strand. Other enzymes, including DNA polymerase I and RNase H, are responsible for removing the RNA primer after DNA polymerase III has begun its work, replacing it with DNA nucleotides (Ogawa & Okazaki, 1984). When these enzymes finish, they leave a nick between the section of DNA that was formerly the primer and the elongated department of Deoxyribonucleic acid. Some other enzyme called Dna ligase then acts to seal the bond between the two adjacent nucleotides.
Deoxyribonucleic acid Polymerase Merely Moves in One Direction
Subsequently a primer is synthesized on a strand of Deoxyribonucleic acid and the DNA strands unwind, synthesis and elongation tin can proceed in but one direction. As previously mentioned, DNA polymerase tin can only add to the 3' end, so the 5' end of the primer remains unaltered. Consequently, synthesis gain immediately only along the then-called leading strand. This immediate replication is known every bit continuous replication. The other strand (in the 5' direction from the primer) is chosen the lagging strand, and replication along it is called discontinuous replication. The double helix has to unwind a bit before the synthesis of another primer can be initiated further upwards on the lagging strand. Synthesis tin then occur from the 3' end of that new primer. Next, the double helix unwinds a bit more than, and another spurt of replication gain. As a upshot, replication along the lagging strand can only proceed in short, discontinuous spurts (Figure 3).
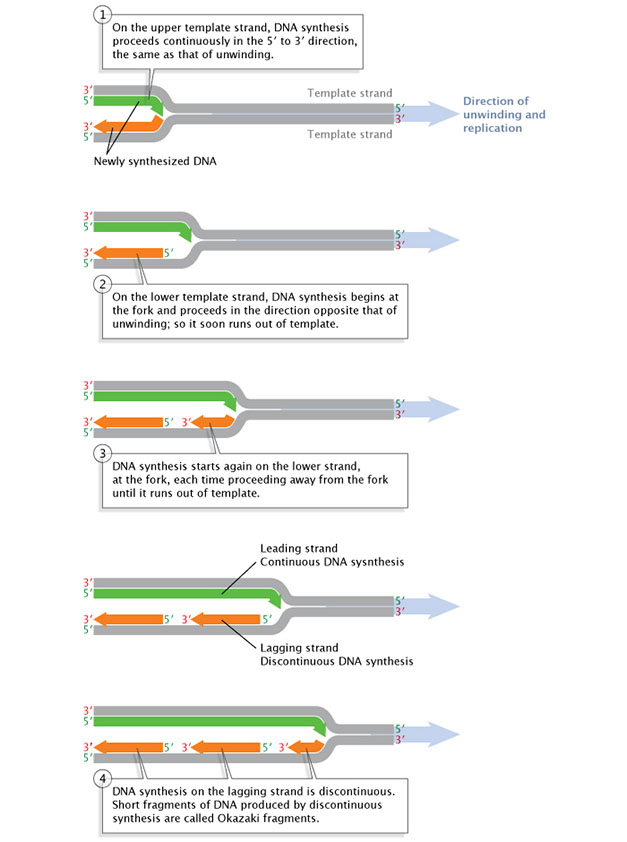
Figure three: Replication of the leading DNA strand is continuous, while replication along the lagging strand is discontinuous.
Afterwards a brusque length of the DNA has been unwound, synthesis must go along in the 5' to 3' direction; that is, in the direction reverse that of the unwinding.
© 2014 Nature Didactics Adapted from Pierce, Benjamin. Genetics: A Conceptual Approach, second ed. All rights reserved. ![]()
The fragments of newly synthesized DNA along the lagging strand are called Okazaki fragments, named in honor of their discoverer, Japanese molecular biologist Reiji Okazaki. Okazaki and his colleagues made their discovery past conducting what is known as a pulse-chase experiment, which involved exposing replicating DNA to a short "pulse" of isotope-labeled nucleotides then varying the length of time that the cells would be exposed to nonlabeled nucleotides. This later menstruation is called the "chase" (Okazaki et al., 1968). The labeled nucleotides were incorporated into growing DNA molecules just during the initial few seconds of the pulse; thereafter, but nonlabeled nucleotides were incorporated during the chase. The scientists and so centrifuged the newly synthesized Dna and observed that the shorter chases resulted in near of the radioactivity appearing in "wearisome" Deoxyribonucleic acid. The sedimentation rate was determined past size: smaller fragments precipitated more slowly than larger fragments considering of their lighter weight. As the investigators increased the length of the chases, radioactivity in the "fast" Dna increased with picayune or no increase of radioactivity in the slow DNA. The researchers correctly interpreted these observations to mean that, with short chases, simply very pocket-sized fragments of Deoxyribonucleic acid were being synthesized along the lagging strand. As the chases increased in length, giving Deoxyribonucleic acid more than fourth dimension to replicate, the lagging strand fragments started integrating into longer, heavier, more rapidly sedimenting DNA strands. Today, scientists know that the Okazaki fragments of bacterial Deoxyribonucleic acid are typically between 1,000 and 2,000 nucleotides long, whereas in eukaryotic cells, they are only about 100 to 200 nucleotides long.
The Challenges of Eukaryotic Replication
Bacterial and eukaryotic cells share many of the aforementioned basic features of replication; for case, initiation requires a primer, elongation is always in the five'-to-3' direction, and replication is ever continuous forth the leading strand and discontinuous along the lagging strand. Only at that place are also important differences between bacterial and eukaryotic replication, some of which biologists are still actively researching in an effort to meliorate sympathize the molecular details. I difference is that eukaryotic replication is characterized past many replication origins (oft thousands), not just one, and the sequences of the replication origins vary widely amid species. On the other manus, while the replication origins for bacteria, oriC, vary in length (from about 200 to ane,000 base pairs) and sequence, except amongst closely related organisms, all bacteria withal have but a single replication origin (Mackiewicz et al., 2004).
Eukaryotic replication also utilizes a dissimilar set of DNA polymerase enzymes (e.chiliad., Dna polymerase δ and DNA polymerase ε instead of DNA polymerase III). Scientists are still studying the roles of the 13 eukaryotic polymerases discovered to appointment. In addition, in eukaryotes, the Deoxyribonucleic acid template is compacted by the manner it winds effectually proteins called histones. This Deoxyribonucleic acid-histone complex, called a nucleosome, poses a unique challenge both for the cell and for scientists investigating the molecular details of eukaryotic replication. What happens to nucleosomes during Dna replication? Scientists know from electron micrograph studies that nucleosome reassembly happens very quickly after replication (the reassembled nucleosomes are visible in the electron micrograph images), but they still do not know how this happens (Annunziato, 2005).
Too, whereas bacterial chromosomes are circular, eukaryotic chromosomes are linear. During circular DNA replication, the excised primer is readily replaced by nucleotides, leaving no gap in the newly synthesized DNA. In contrast, in linear DNA replication, there is always a small-scale gap left at the very stop of the chromosome because of the lack of a 3'-OH group for replacement nucleotides to bind. (As mentioned, Deoxyribonucleic acid synthesis can proceed only in the five'-to-three' management.) If there were no way to fill this gap, the DNA molecule would get shorter and shorter with every generation. However, the ends of linear chromosomes—the telomeres—have several properties that preclude this.
DNA replication occurs during the S phase of cell division. In E. coli, this means that the entire genome is replicated in but 40 minutes, at a pace of approximately 1,000 nucleotides per second. In eukaryotes, the pace is much slower: about forty nucleotides per second. The coordination of the protein complexes required for the steps of replication and the speed at which replication must occur in order for cells to carve up are impressive, especially considering that enzymes are also proofreading, which leaves very few errors behind.
Summary
The report of Deoxyribonucleic acid replication started almost as soon equally the structure of Dna was elucidated, and it continues to this day. Currently, the stages of initiation, unwinding, primer synthesis, and elongation are understood in the virtually basic sense, simply many questions remain unanswered, particularly when it comes to replication of the eukaryotic genome. Scientists take devoted decades to the study of replication, and researchers such as Kornberg and Okazaki accept fabricated a number of of import breakthroughs. Nevertheless, much remains to be learned most replication, including how errors in this process contribute to human disease.
References and Recommended Reading
Annunziato, A. T. Separate conclusion: What happens to nucleosomes during Dna replication? Journal of Biological Chemistry 280, 12065–12068 (2005)
Bessman, M. J., et al. Enzymatic synthesis of deoxyribonucleic acrid. Ii. General backdrop of the reaction. Periodical of Biological Chemistry 233, 171–177 (1958)
Kornberg, A. The biological synthesis of deoxyribonucleic acid. Nobel Lecture, December 11, 1959. (link to transcript)
———. Biological synthesis of deoxyribonucleic acid. Science 131, 1503–1508 (1960)
Lehman, I. R., et al. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. Journal of Biological Chemistry 233, 163–170 (1958)
Losick, R., & Shapiro, L. DNA replication: Bringing the mountain to Mohammed. Science 282, 1430–1431 (1998)
Mackiewicz, P., et al. Where does bacterial replication start? Rules for predicting the oriC region. Nucleic Acids Research 32, 3781–3791 (2004)
Ogawa, T., & Okazaki, T. Function of RNase H in DNA replication revealed by RNase H defective mutants of Escherichia coli. Molecular and General Genetics 193, 231–237 (1984)
Okazaki, R., et al. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proceedings of the National Academy of Sciences 59, 598–605 (1968)
harperaptaidene68.blogspot.com
Source: http://www.nature.com/scitable/topicpage/major-molecular-events-of-dna-replication-413